

The molality of a solution is the number of moles of solute per kilogram of solvent. The molarity of a solution is the number of moles of solute per liter of solution. Thus, one mole of sodium chloride weighs 58.44 grams. For example, the molecular weight of sodium chloride is 58.44.

One mole is defined as the gram molecular weight of the solute. The unit for measuring solutes is the mole. In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are measured.
Active transport requires energy in the form of ATP conversion, carrier proteins, or pumps in order to move ions against the concentration gradient.Ĭoncept of Osmolality and Milliequivalent Facilitated diffusion requires protein-based channels for moving the solute. This movement can be accomplished by facilitated diffusion and active transport. If electrolyte ions could passively diffuse across membranes, it would be impossible to maintain specific concentrations of ions in each fluid compartment therefore they require special mechanisms to cross the semi-permeable membranes in the body. Water can pass through membranes by passive diffusion. Because electrolytes dissociate into their component ions, they, in essence, add more solute particles into the solution and have a greater effect on osmotic pressure, per mass than compounds that do not dissociate in water, such as glucose. The number of solute atoms or molecules and not dependent on the size of the solute molecules. Osmotic pressure is influenced by the concentration of solutes in a solution. For this reason, athletes are encouraged to replace electrolytes and fluids during periods of increased activity and perspiration.
Electrolytes are lost from the body during urination and perspiration. Magnesium (Mg +2), and the anions chloride (Cl –), carbonate (CO 3 -2), bicarbonate (HCO 3 –), and phosphate(PO 3 –). The most important ions, whose concentrations are very closely regulated in body fluids, are the cations sodium (Na +), potassium (K +), calcium (Ca +2), In water, sodium chloride (NaCl), dissociates into the sodium ion (Na +) and the chloride ion (Cl –). Transport of Electrolytes across Cell MembranesĮlectrolytes, such as sodium chloride, ionize in water, meaning that they dissociate into their component ions. The blood maintains an isotonic environment so that cells neither shrink nor swell. In a hypotonic environment, cells tend to swell due to intake of water. Cells placed in a hypertonic environment tend to shrink due to loss of water. Isotonic cells have an equal concentration of solutes inside and outside the cell this equalizes the osmotic pressure on either side of the cell membrane which is a semi-permeable membrane. A cell placed in a solution with higher salt concentration, on the other hand, tends to make the membrane shrivel up due to loss of water into the hypertonic or “high salt” environment. As seen in Figure 22.2, a cell placed in water tends to swell due to gain of water from the hypotonic or “low salt” environment. Solutions on two sides of a semi-permeable membrane tend to equalize in solute concentration by movement of solutes and/or water across the membrane. Semi-permeable membranes are permeable (or permissive) to certain types of solutes and water. The membranes of the body (such as the pleural, serous, and cell membranes) are semi-permeable membranes. The body’s fluids include blood plasma, the cytosol within cells, and interstitial fluid, the fluid that exists in the spaces between cells and tissues of the body. Both electrolytes and non-electrolytes contribute to the osmotic balance. A non-electrolyte, in contrast, doesn’t dissociate into ions during water dissolution. An electrolyte is a solute that dissociates into ions when dissolved in water.
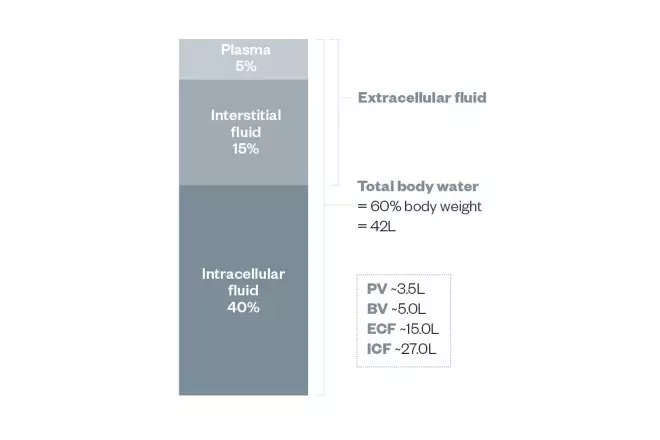
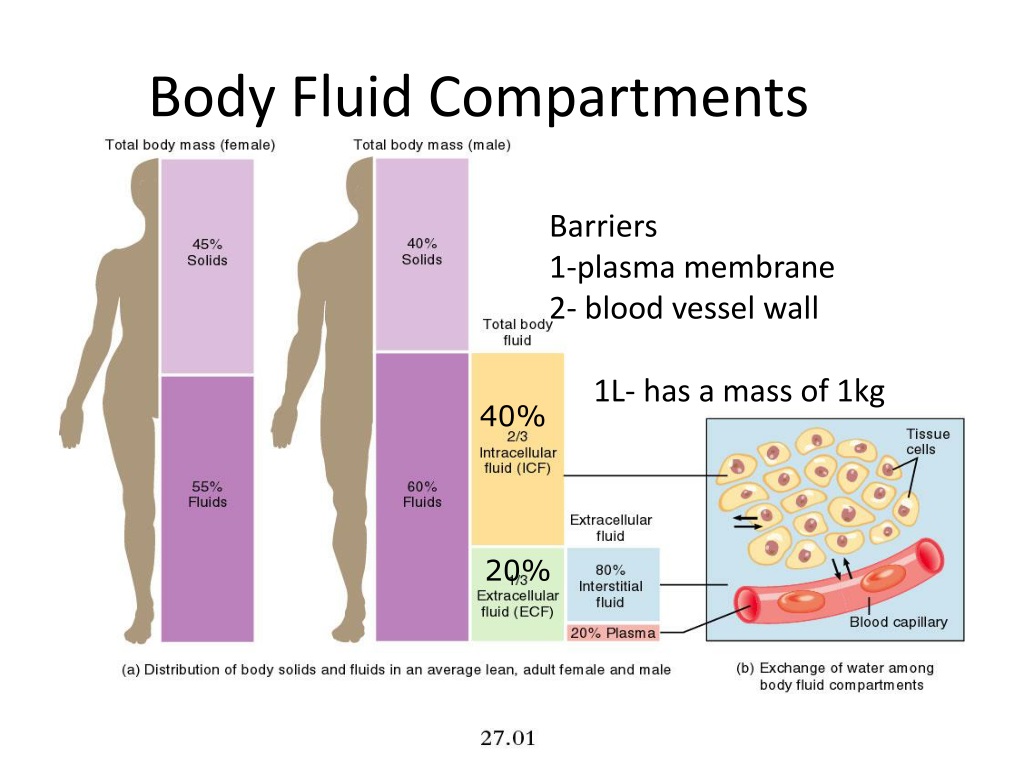
Figure 5.1c body fluid compartments plus#
Osmoregulation is the process of maintenance of salt and water balance ( osmotic balance ) across membranes within the body’s fluids, which are composed of water, plus electrolytes and non-electrolytes. Osmosis is the diffusion of water across a membrane in response to osmotic pressure caused by an imbalance of molecules on either side of the membrane.


 0 kommentar(er)
0 kommentar(er)
